Cell cryopreservation tips and tricks for 2022

It’s not easy to grow cells. It’s not easy to take care of them. They’re like really troublesome toddlers. There’s a lot that can go wrong, even when you think things are going great.
But, unlike toddlers, you can vastly reduce the trouble your cells get into by freezing them.
You stop the biological clock and preserve them in the state you want.
This guide can serve as a reminder:
- About the steps you should take your time with.
- About the small details in your equipment selection that make a big difference in cell recovery.
- About the best way to be frugal with your budget without giving up quality.
So bookmark this page and come back often. We’ll update it any time valuable information comes our way.
Why do we freeze cells?
When they’re frozen and tucked away, cell cultures need no maintenance at all.
There’s a cost - either running your -130°C freezer or maintaining your liquid nitrogen supply.
But the cost of keeping cells frozen vastly ourweighs the cost of maintaining actively growing cultures. And even moreso the cost of getting a new culture from a biobank.
Cellular changes happen in all actively growing populations. These changes usually result in the loss of important characteristics. They introduce unwanted variables into long-term experiments.
Cryogenically preserved cultures do not undergo any detectable changes once they are stored below -130°C. Frozen cultures, then allow your long term experiments to be successfully completed without these unwanted variables. Frozen cultures also provide a valuable baseline against to compare future experimentally-induced changes against.
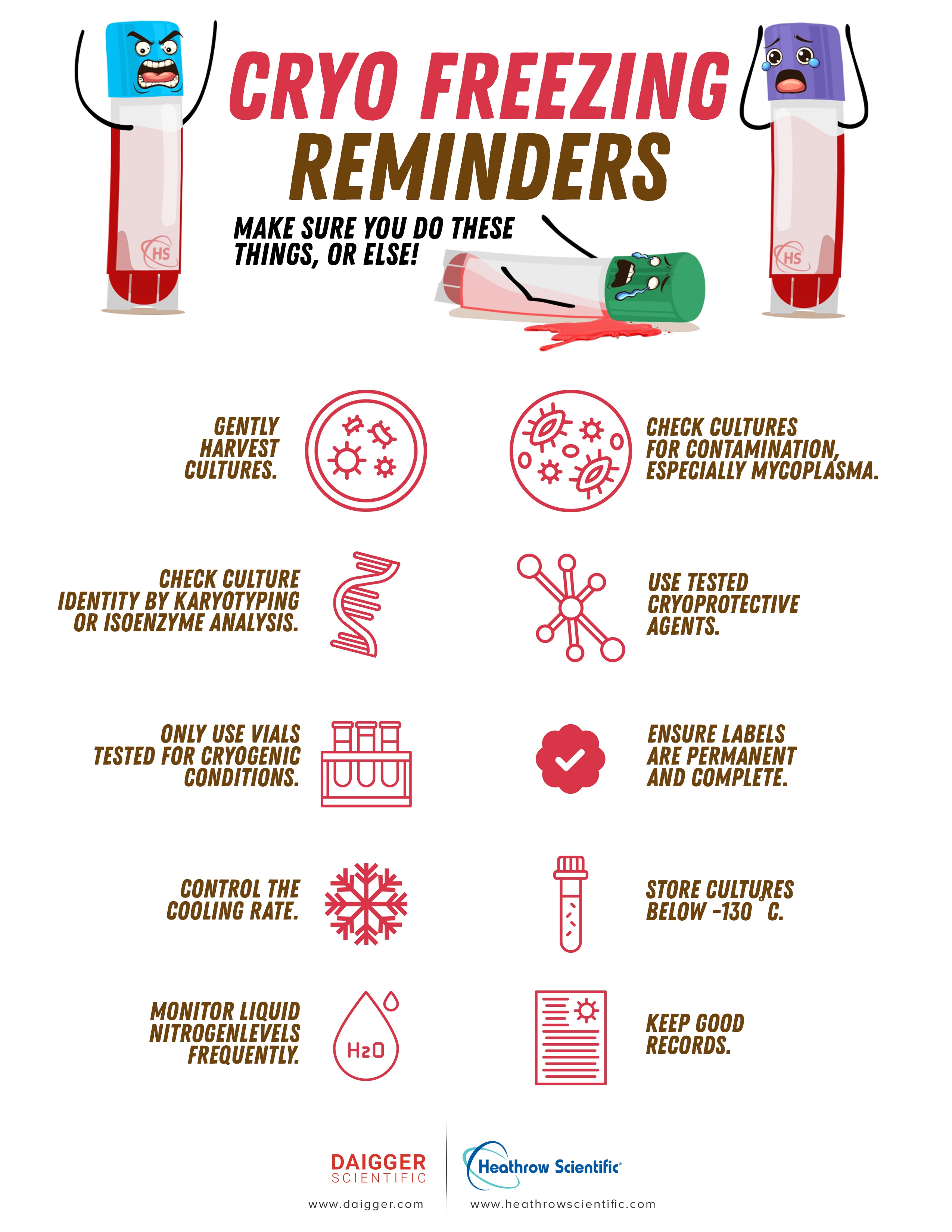
How to get cryopreservation of cells right
The cooling and freezing rate has to be just right.
Cooling from room temperature to 0°C slows cellular metabolism. As long as the culture medium is osmotically balanced, there’s no cellular damage.
From around 0˚C to -20˚C, ice crystals begin to form in the extracellular space. Water begins to move out of the cells and into the partially frozen extracellular medium. The cells are starting to dehydrate and shrink.
If this process went down quickly, ice crystals would form inside the cells. The cells would die during thawing.
But slowing the cooling process pulls water from the cells. No water, no ice crystals.
It’s not easy to control manually. So something like a Heathrow Scientific Cool Container helps operators stay in control.
Stuff that impacts cryopreservation
Cell selection
Choose strong cells to work with.
Look for signs of contamination - fungi, bacteria, mycoplasmas, bacterial.
Make sure you change their medium 24 hours before harvesting.
Harvesting cells
Start with the usual harvesting procedure recommended for your culture. Be over-the-top-gentle.
To make sure your final frozen stock is consistent, pool the contents of your cryovials.
This also makes it easier to perform quality control testing for microbial contamination and culture identity.
Keep the cells chilled to slow their metabolism and prevent cell clumping.
Cryoprotection
Cryoprotection is necessary to minimize or prevent the damage that still comes with slow freezing.
Common cryoprotective agents include:
- Methyl acetamide
- Methyl alcohol
- Ethylene glycol
- Polyvinyl pyrrolidone
- Dimethylsulfoxide (DMSO)
- Glycerol
DMSO and glycerol are probably used in your lab.
Always use reagent or other high purity grades that have been tested for suitability.
Storing at super low temperatures
Because temperatures in cryogenic storage are incredibly low, not all vessel materials or designs are suitable or safe. Most materials are brittle at these temperatures. Vessels that aren’t explicitly made for these low tempeartures will crack and your samples, and the time you spend preparing them, will be wasted.
We probably don’t have to say this, but please don’t immerse plastic or glass cryogenic vials in liquefied gases. If you do, they can start to leak. Then, when you return them to room temperature, pressure builds quickly and shatters the vials or cap seals. Always store vials in the vapor phase above any liquefied gas (you know, but we said it anyway).
Vessels for super low temperatures
You’re going use polypropylene screw-capped vials. You can get them from about 1ml to 5ml, but generally you stick to 2ml for cryogenic storage unless you have a unique application.
These Heathrow Scientific externally-threaded cryo vials are priced about 30% more affordably than comparable vials from VWR or other large manufacturers. And they’re available right now(while other brands are 12+ weeks backlogged on orders of cryo vials).
Externally threaded vials reduce the possibility of contamination, and are generally the preferred choice for cryogenic storage.
Your lab may have used glass ampules in the past. But sealing and labeling problems have made glass ampules largely irrelevant in cell culture labs.

Keeping track
A cryogenic cell repository will outlast the lab workers who contribute to it. So recordkeeping and labeling is extremely important.
Labels usually contain information like:
- The culture’s identity
- Frozen date
- Initials of the person responsible
Heathrow Scientific cryo vials have areas for easy labeling. Other vials and ampules without marking spots require cloth labels with special adhesives.
In a lab notebook like this water and chemical-resistant lab notebook, fully detail in the records the culture’s storage conditions, including all of the following information:
- Culture identity
- Passage or population doubling level
- Date frozen
- Freezing medium and method used
- Number of cells per vial
- Total number of vials initially frozen and the number remaining,
- Locations
- Expected viability
- Results of all quality control tests (sterility,mycoplasma, species, karyotype, etc.).
Additional culture information, especially their origin, history, growth parameters, special characteristics, and applications, is also helpful and should be included whenever possible.
Make special efforts to keep all records up-to-date and to ensure everyone uses them correctly.
A refresher about cooling rate
The cooling has to be just slow enough to allow the cells time to dehydrate, but speedy enough to prevent as much dehydration damage as possible.
A cooling rate of -1°C to -3°C /minute is plenty for most animal cell cultures.
Some manufacturers will tell you the best way to control cooling rates is using electronic programmable freezing units. They’ll say the results are more consistent than less technical methods.
In the lab, however, low tech cooling methods are often the standard.
Another design uses a media-filled container with a rack to achieve controlled cooling. Something like:
The container is filled with alcohol or a gel, where that acts as a bath to achieve reliable heat transfer and cooling. After freezing 4 to 5 hours, the vials are removed from the container and transferred to the cryo storage location.
When it’s time to thaw
Remove the vial from its storage location and carefully check both the label and storage record to ensure that it is the correct culture.
Place the Heathrow Scientific cryo vial in warm water and agitate gently until it’s thawed entirely.
Fast thawing of about 1 minute or 1:30 at 37°C provides the best recovery for most cell cultures
Sometimes you have a problem
Viability issues are easy to identify after cultures are thawed and plated.
Generally, a problem rear its head during one of four phases of the process.
- During cell harvesting and processing
Problems may be caused by toxic cryoprotectant, leaving cell suspensions too long at room temperature, letting them sit too long at a highly-basic pH. - During the cooling (freezing) process
Cell damage and reduced viability come from cooling too quickly or too slowly. Maybe from interrupting the cooling process. - In cryogenic storage
Culture viability is often reduced when vials are allowed to warm up during transfer to the freezer, or if the repository temperature is not consistently maintained at appropriate cryogenic temperatures. - While thawingThere can be problems when the thawing process is too slow or the cryoprotectants are improperly removed.
Start with cryovials, and make sure you have the best tools for your job
Working with cells in super cold conditions is a finicky job. Sometimes you can do everything right and still end up with a damaged cells and wasted days.
You only have the power to do two things.
- Follow the best practices for working with your samples.
- Do your best to equip yourself with the most appropriate tools.
The tools you need are here. From cryo vials to cardboard cryovial boxes, coolers, containers, notebooks, and even the simple timer so you don’t have to use your phone when cooling down samples.
Login to order with your preferred pricing, or get in touch with our team to set your organization’s special pricing up today.
Sign in here for your preferred pricing and order your cryovials today